ACID & ALKALINE BALANCE - ASSESSING pH
Over acidity, which can become a dangerous condition that weakens all body systems, is very common today. It gives rise to an internal environment conducive to disease, as opposed to a pH-balanced environment which allows normal body function necessary for the body to resist disease. A healthy body maintains adequate alkaline reserves to meet emergency demands. When excess acids must be neutralized, our alkaline reserves are depleted leaving the body in a weakened condition.
The concept of acid alkaline imbalance as the cause of disease is not new. In 1933 a New York doctor named William Howard Hay published a ground-breaking book, A New Health Era in which he maintains that all disease is caused by autotoxication (or "self-poisoning") due to acid accumulation in the body:
Now we depart from health in just the proportion to which we have allowed our alkalies to be dissipated by introduction of acid-forming food in too great amount... It may seem strange to say that all disease is the same thing, no matter what its myriad modes of expression, but it is verily so. William Howard Hay, M.D.
More recently, in his remarkable book Alkalize or Die (see recommended reading), Dr. Theodore A. Baroody says essentially the same thing:
The countless names of illnesses do not really matter. What does matter is that they all come from the same root cause...too much tissue acid waste in the body! Theodore A. Baroody, N.D., D.C., Ph.D.
Understanding pH
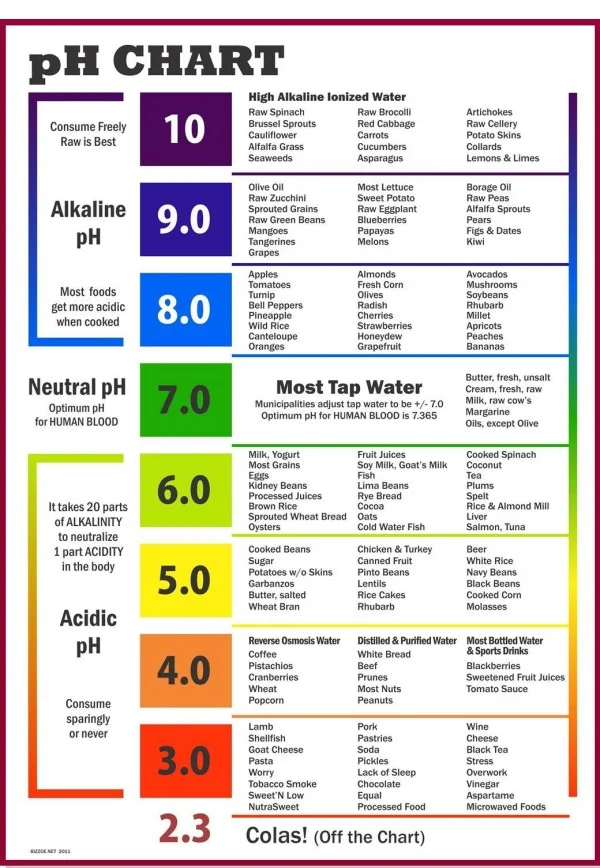
pH (potential of hydrogen) is a measure of the acidity or alkalinity of a solution. It is measured on a scale of 0 to 14—the lower the pH the more acidic the solution, the higher the pH the more alkaline (or base) the solution. When a solution is neither acid nor alkaline it has a pH of 7 which is neutral.
Water is the most abundant compound in the human body, comprising 70% of the body. The body has an acid-alkaline (or acid-base) ratio called the pH which is a balance between positively charges ions (acid-forming) and negatively charged ions (alkaline-forming.) The body continually strives to balance pH. When this balance is compromised many problems can occur.
It is important to understand that we are not talking about stomach acid or the pH of the stomach. We are talking about the pH of the body's fluids and tissues which is an entirely different matter.
Test Your Body's Acidity or Alkalinity with pH Strips
It is recommended that you test your pH levels to determine if your body's pH needs immediate attention. By using pH test strips, you can determine your pH factor quickly and easily in the privacy of your own home. If your urinary pH fluctuates between 6.0 to 6.5 in the morning and between 6.5 and 7.0 in the evening, your body is functioning within a healthy range. If your saliva stays between 6.5 and 7.5 all day, your body is functioning within a healthy range. The best time to test your pH is about one hour before a meal and two hours after a meal. Test your pH two days a week.
Urine pH
The results of urine testing indicate how well your body is assimilating minerals, especially calcium, magnesium, sodium and potassium. These are called the "acid buffers" because they are used by the body to control the acid level. If acid levels are too high, the body will not be able to excrete acid. It must either store the acid in body tissues (autotoxication) or buffer it—that is, borrow minerals from organs, bones, etc. in order to neutralize acidity.
Saliva pH
You'll also want to test the pH of your saliva. The results of saliva testing indicate the activity of digestive enzymes in your body, especially the activity of the liver and stomach. This reveals the flow of enzymes running through your body and shows their effect on all the body systems. Some people will have acidic pH readings from both urine and saliva—this is referred to as "double acid."
Keeping the Balance Right for Excellent Health
Your body is able to assimilate minerals and nutrients properly only when its pH is balanced. It is therefore possible for you to be taking healthy nutrients and yet be unable to absorb or use them. If you are not getting the results you expected from your nutritional or herbal program, look for an acid alkaline imbalance. Even the right herbal program may not work if your body's pH is out of balance.
What Causes Me to be Acidic?
The reason acidosis is more common in our society is mostly due to the typical Western diet, which is far too high in acid-producing animal products like meat, eggs and dairy, and far too low in alkaline-producing foods like fresh vegetables. Additionally, we eat acid-producing processed foods like white flour and sugar and drink acid-producing beverages like coffee and soft drinks. We use too many drugs, which are acid-forming; and we use artificial chemical sweetners like NutraSweet, Equal, or aspartame, which are extremely acid-forming. One of the best things we can do to correct an overly-acid body is to clean up the diet and lifestyle.
Most people who suffer from unbalanced pH are acidic. This condition forces the body to borrow minerals—including calcium, sodium, potassium and magnesium—from vital organs and bones to buffer (neutralize) the acid and safely remove it from the body. Because of this strain, the body can suffer severe and prolonged damage due to high acidity—a condition that may go undetected for years.
Mild acidosis can cause such problems as:
•Cardiovascular damage, including the constriction of blood vessels and the reduction of oxygen.
•Weight gain, obesity and diabetes.
•Bladder and kidney conditions, including kidney stones.
•Immune deficiency.
•Acceleration of free radical damage, possibly contributing to cancerous mutations.
•Premature aging.
•Osteoporosis; weak, brittle bones, hip fractures and bone spurs.
•Joint pain, aching muscles and lactic acid buildup.
•Low energy and chronic fatigue.
A recent seven-year study conducted at the University of California, San Francisco, on 9,000 women showed that those who have chronic acidosis are at greater risk for bone loss than those who have normal pH levels. The scientists who carried out this experiment believe that many of the hip fractures prevalent among middle-aged women are connected to high acidity caused by a diet rich in animal foods and low in vegetables. This is because the body borrows calcium from the bones in order to balance pH. — American Journal of Clinical Nutrition
Source: Prevent Disease